B1. Methods of Stem Cells Identification: Stem cells live with other stem cells so we need some methods to identify them from the other normal and differentiated cells. A few methods utilized for the identification of stem cells are discussed below:
Scientific work on the morphology and size of Mesenchymal stem cells (MSC) indicates that stem cells can change from small, spindle-like cells in the early passage to large and polygonal types in later passages. After a week in culture, fibroblast-like cells can form. Usually, stem cells have high nuclear to cytoplasmic ratio than differentiated cells. Stem cells grow in colonies and the distance between cells and the colonies is unique to stem cells. For example, the morphology of human ESCs, mean nuclei area, and distance between neighbors can be good indicators to detect the best colonies of stem cells. The size of hESCs colonies differs between 0.1-1.1 mm2 for 4 days in culture (Orozco-Fuentes et al., 2019) (Figure 4).
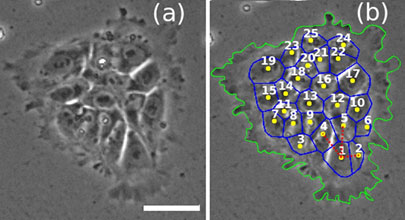
Figure 4: Colonies of 25 cells of hESCs showing the first cell (labeled 1) along with neighbors (labeled 2-25) (Orozco-Fuentes et al., 2019).
Based on the expression of protein markers - Typically, MSCs and associated progenitor cells are detected using specific cluster of differentiation (CD) markers. The International Society for Cellular Therapyrecommends that MSC-containing cell preparations should have cells that express at least CD105, CD90, and CD44 as positive markers and CD34, CD45, CD14 or CD11b, C79α or CD19 and HLA-DR as the negative markers. Nuclear expression of pluripotency transcription factors like OCT4, SOX2, Nanog, c-Myc, KLM4, and others are taken as markers of ESCs. Likewise, the expression of different marker proteins specifies tissue-specific stem cell. Ffor example, NRF2, FNDC3B, and NUP153 are the markers of bovine mammary stem and progenitor cells (Choudhary and Capuco, 2021).
Another technique of identification of tissue-specific stem cells is the label retention study. Long-term bromodeoxyuridine label-retaining cells (LRC) have been identified in rat brains sweat glands, kidneys, intestine, bovine mammary gland and many other organs. When shown independently to be actively cycling, the retention of previously-labeled DNA strands is attributed to non-random chromosome segregation, a specific property of asymmetrically self-renewing tissue stem cells (R. K. Choudhary et al., 2013). Label retention studies involve the incorporation of BrdU, a thymidine analog, into the DNA of dividing cells. BrdU is actively taken up by cells during DNA synthesis (S phase) and becomes incorporated into their genomic DNA. By analyzing the presence or absence of the label in specific cell populations, label retention studies can help identify and distinguish stem cells from other cell types.
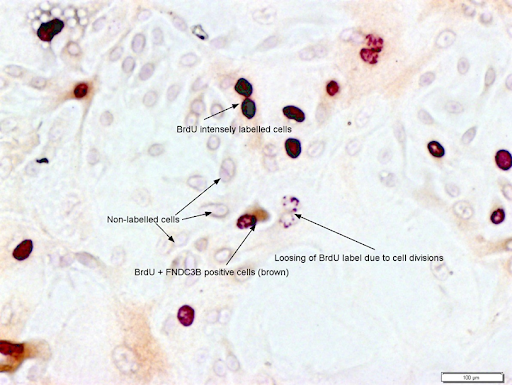
Figure 5: BrdU labeling og epithelial cells after three weeks of chase period showing varied intensity of nuclear labeling. Stem cells, known for their slow division rates, retain the labeled BrdU over (intensely labeled cells) for an extended period, while more rapidly dividing cells lose the label through dilution during successive divisions (shwon by punctate nuclear staining). BrdU labeled cells co-stained with FNDC3B, a marker of mamamry epithelial cells, has also been shown. (Photo credit: Ratan Choudhary, unpublished photomicrograph).
Multiparameter flow cytometry and fluorescence-activated cell sorting (FACS) are used to detect and isolate stem cells and progenitor cells based on the expression of cell surface markers. The method is similar to immunohistochemistry (IHC), wherein harvested cells are labeled at a single-cell level, and their marker-specific fractions can be separated from the heterologous cell population. Representative cell surface markers of selected ESCs, iPSCs, hematopoietic stem cells (HSCs), and mesenchymal stem cells (MSCs) of humans, mice, dogs, and bovines are given in the table. ESCs and iPSCs, expression of pluripotency transcription factors (OCT4, SOX2, Nanog, c-Myc, KLM4) is considered markers.
| Species | ESCs and iPSCs | HSC | MSC |
|---|---|---|---|
Humans |
Positive markers: Alkaline Phosphatase, SSEA-4, SSEA-3, TRA-1-81, TRA-1-60 Negative markers: SSEA-1 |
Lin– CD34+CD38– CD90+CD45RA– CD49f+ |
Positive markers: CD44, CD73, CD90, CD105 |
|
Mice |
Positive markers: Alkaline Phosphatase, SSEA-1, SSEA-4, SSEA-3, TRA-1-81, TRA-1-60 |
Positive markers: CD150, CD117, Sca1 Negative markers: CD34, CD41, CD48, Lineage |
Positive markers: CD29, CD44, CD90, CD105, CD106, Sca-1 Negative markers: CD11b, CD31, CD45, Ter-119 |
|
Dogs |
Positive markers: SSEA-3, SSEA-4, TRA1– 60, TRA-1– 81, and alkaline phosphatase Low level: SSEA-1 |
Positive markers: CD34, CD117, and CD45 |
Positive markers: CD29, CD44, CD90 and CD105 |
|
Bovines |
Positive markers: SSEA-4, alkaline phosphatase Negative markers: SSEA1, TRA-1-60 and TRA-1-81 |
Positive markers: CD34, CD90 and CD117 |
Positive markers: CD29, CD166, CD105, CD73, CD44, CD90 Negative markers: CD14, CD31, CD34, CD45, CD117, CD80, CD86, CD106, MHC-II and pan-cytokeratin |
*Human lineage (lin) markers: CD2, CD3, CD4, CD7, CD8, CD10, CD11b, CD14, CD19, CD20, CD56, CD235a
In vitro methods of stem cell identification involve laboratory techniques and assays to characterize and identify stem cells based on their specific properties and markers. They includes, 1) Mophorlogical observations, 2) Expression of stem cell markers using immunocytochemistry, 3) Flow cytometry; 4) Gene expressoin analysis by assessing expression of stem cell markers, 5) Estimation of telomerase enzyme activity, and 5) Functional assay like differentiation ability and sperioid formation. Sphere formation assay of stem cells and cancer stem cells is an in vitro method to access the self-renewal ability of stem cells present in the culture. Formation of mammosphere from mammary stem cells, neurosphere from neuronal stem cells, and tumor sphere from cancer stem cells.
KCS involves monitoring the increase in cell numbers in a culture through sequential measurements taken at different time points. This technique provides valuable information about stem cell proliferation rates and can be used to assess their expansion potential or response to specific treatments. Here's a general description of the kinetic stem cell counting technique: This is a recently developed technique for precisely quantifying tissue stem cells in complex tissue cell preparations (Sherley et al., 2022). KSC counting can be used to determine the respective percentages of HSCs (blue line), progenitor cells (red line), and terminally differentiated cells (green line) in human blood cell preparations during ten 3-day serial culture passages (Figure 5).
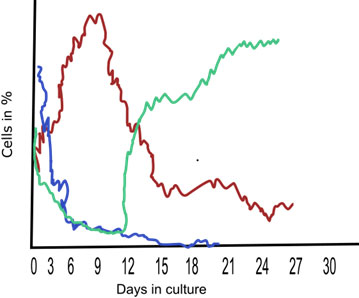
Figure 6: A sketch depicting the results of the KCS counting method for the determination of stem cells (blue line), progenitor cells (red line), and terminally differentiated cells (green line) over the period in culture.
The mechanisms of stem cell action are complex and multifaceted, and they can vary depending on the specific type of stem cell and the context in which they are applied. However, there are several general mechanisms through which stem cells exert their effects. Here are some key mechanisms of stem cell action:
Differentiation: Proliferation and differentiation of tissue stem cells to replace damaged cells: Cells divide by symmetric or asymmetric cell kinetics programs. Symmetric division expands the stem cell pool, whereas asymmetric division maintains a constant number. Stem cell divides to produce transiently-amplifying progenitor cells, which differentiate into functional and terminally-differentiated cells (Figure 6). Ultimately, depending on the environmental cues, stem cells expand to increase inside the tissue and maintain tissue homeostasis (Figure 7).
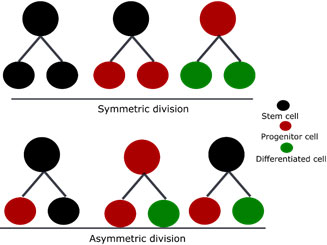
Figure 7: Symmetric and asymmetric division of tissue stem cell resulting in two daughter cells of similar or dissimilar types.
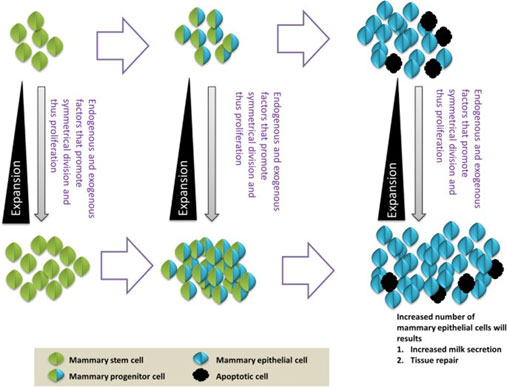
Figure 8: Expansion of stem and progenitor cells which ultimately produce terminally differentiated cells (R. Choudhary, 2014).
Paracrine signaling – The paracrine action of stem cells has emerged as one of the mechanisms of stem cell-mediated therapeutic applications. Soluble factors released from stem cells may act on damaged neighbor cells mediated through microvesicles (M.V.s). M.V.s are loaded with proteins, peptides, miRNAs, bioactive lipids, and other second messenger molecules are transferred to injured cells that reprogram damaged cells to restore functionality or maybe even regain stem cell-like phenotype (Figure 8).
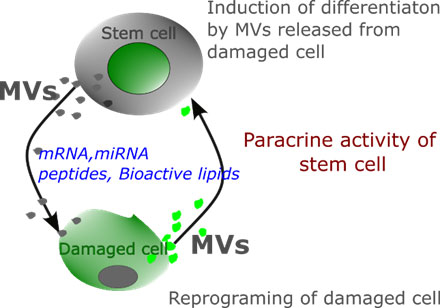
Figure 9: Paracrine signaling. Microvesicles released from stem cells repairs injured cells and restore their functions. It has also been postulated that microvesicles released by the damaged cells may induce differentiation of stem cells and thus maintain cell turnover.
Immunomodulation: Stem cells possess immunomodulatory properties, meaning they can influence the immune response. They can suppress immune reactions, reduce inflammation, and promote tissue healing by interacting with immune cells and modulating their activity. Stem cells can interact with various immune cells, such as T cells, B cells, macrophages, and dendritic cells, through direct cell-to-cell contact or through the secretion of soluble factors or transfer cell organelles or molecules through tunneling nanotubes (TNT). Interaction of molecules with immune cells results in (i) Suppression of inappropriate or excessive immune responses, (ii) Reduction of inflammation, (iii) polarization of immun cells like development of regulatory T-cells or Tregs, and others like promotion of tissue healing.
Tunneling nanotubes (TNT) formation: TNT are considered a novel cargo route between the cell through which subcellular organelles and secreted factors (secretomes) are transferred (Figure 9). For example, hemopoietic progenitor cells are connected via F-actin-dependent TNT, and the stem cell marker CD133 is transferred between hemopoietic cells. In another study, TNT-mediated mitochondria transfer was observed between bone marrow-derived mesenchymal stem cells and pulposus cells of the brain (Yang et al., 2022).
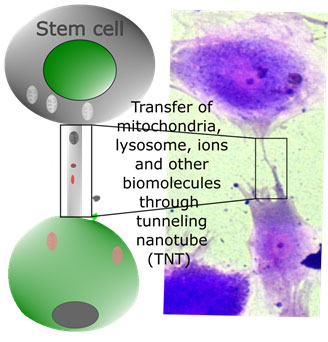
Figure 10: Exchange of cell organelles and biologically active lipids and peptides between stem cells and neighboring cells through tunneling nanotubes.
Cell replcement: By differentiating into the appropriate cell types, stem cells can integrate into the damaged tissue and restore its structure and function. When stem cells are introduced into a damaged tissue or organ, they can integrate into the existing cellular infrastructure and undergo differentiation based on the surrounding signals and cues. For example, MSCs differentiate into various cell types such as osteoblasts (bone cells), chondrocytes (cartilage cells), and adipocytes (fat cells) dependig upon the signal of diffeentiotion. Stem cells can also integrate into the damaged tissue and restore its structure and function. In a study conducted using human ESCs, stem cells fused with fibroblasts resulted in hybrid cells, which have the typical characteristics of ESCs. The result established that ESCs could reprogram other cells to an embryonic-like stateClick or tap here to enter text.. Later, a fusion of mesenchymal stem cells with tumor cells was observed, and mechanistic molecular regulation of fusion events was elucidated (Zhang et al., 2021).
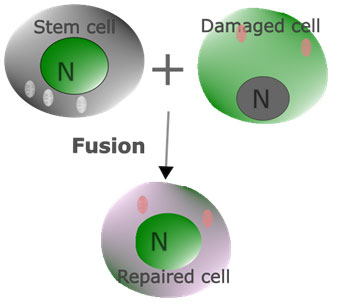
Figure 11: The fusion of stem cells with neighboring cells for restoring the function of damaged cells has also been a postulate for the mechanisms of stem cell action.
Tissue protection and anti-apoptosis: Stem cells have the ability to protect existing cells from injury and cell death. Apoptotic bodies of stem cells engulfed by the macrophage to induce cytokine production – Apoptosis is necessary for exerting the immunomodulatory functions of stem cell therapy. It has been observed recently that apoptotic mesenchymal stem cells, after intravenous injection, were found in the lungs of mice. An alveolar macrophage of the lungs engulfed apoptotic bodies of stem cells and released cytokine -IL10 through phosphatidylserine-recruited macrophage that acted on neutrophils and natural killer cells (Figure 11). Click or tap here to enter text.Investigators concluded that MSC apoptosis and phagocytosis by the macrophage are pivotal in their therapeutic efficacy (Pang et al., 2021).
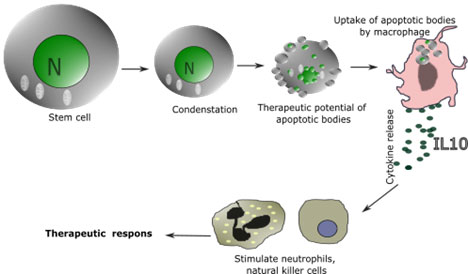
Figure 12: Apoptosis, or programmed cell death, has been considered the significant step in inducing epithelial cell proliferation and hence a strategic player in tissue regeneration. Recent studies suggest apoptosis of mesenchymal stem cells in immunosuppression and required for therapeutic functions.
- Apoptosis- A process of programmed cell death mediated by intrinsic and extrinsic factors
- Asymmetric Cell Division –Cell divisions that produce two different types of cells.
- Asymmetric Self-Renewal Division – The cell division process by which a tissue stem cell produces a new tissue stem cell and a differentiated progenitor cell.
- Blastocyst – After successful fertilization, the zygote divides rapidly and makes a rapidly dividing ball of cells called the blastocyst. It contains ‘inner cell mass’ or ICM and trophoblasts that surround the inner cell mass for nourishment. The ICM is the source of embryonic stem cells (ESCs).
- Blood Cells – Various types of cells derived from hematopoietic stem cells (HSC) are called blood cells. Different blood cells derived from myeloid progenitors are megakaryocytes, erythrocytes, mast cells, and megablast. Megablasts further give rise to basophils, neutrophils, eosinophils, and monocytes (called macrophages in tissue). Other blood cells derived from lymphoid progenitors are natural killer cells, T-lymphocytes, B-lymphocytes, and plasma cells.
- Bone Marrow – The spongy tissue found inside the long bones is called bone marrow. Bone marrow is the reservoir of mesenchymal stem cells (MSCs) called bone marrow-derived MSCs. It is also a major source of HSCs, which can be mobilized by specific factors to enter the peripheral bloodstream.
- Cancer – A disease that is associated with the abnormal growth of cells or tissue that has the potential to invade other tissue. A mass of cancer cells is called a tumor. The process of oncogenesis is hypothesized to be mediated through cancer stem cells.
- Cardiovascular – Related to heart and blood vessels.
- Cell –The biological living unit of large-bodied organisms like humans. Trillions of cells multiply and change into different forms and structures that integrate to make the organs and tissues of multicellular plants and animals.
- Cell Division – The process by which cells multiply by growing in mass, duplicating their DNA, and splitting into two new cells.
- Cell Kinetics – Processes that result in changes in cell number.
- Chimera – Presence of two types of cells derived from two different individuals of the same or even from another species. A chimera is created when pluripotent stem cells of another embryo of the same or different species are added to another developing embryo.
- Chromosome – Densely packed structures that contain a cell’s nuclear DNA. Normal human cells have 46 chromosomes.
- Chromosome segregation – The process by which dividing cells place an equal number of chromosomes into each new cell produced.
- Congenital Arthritis – Joint defects that are deformed at birth
- Cytokines – Cell signaling molecules (peptides) released from various immune and non-immune cells that affect (enhance or slow down) the body's immune system.
- Differentiation – A process of changing stem cells to more specialized cell types. The production of progenitor cells by stem cells and then the formation of terminally-differentiated cells from progenitor cells are the events of tissue cell differentiation.
- ESC – Embryonic stem cells are the cells derived from the inner cell mass of the blastocyst. ESCs are pluripotent.
- Fibroblast – A type of differentiated cell often used for reprogramming cells toward the generation of induced pluripotent stem cells (iPSCs) in stem cell biology.
- Gene – The basic unit of heredity and the part of DNA that codes for proteins.
- Genetic Medicine – These genetic materials, DNA or RNA, are delivered into the body as therapeutics.
- Genome - Entire genetic material of an organism or a cell. It includes all nuclear DNA plus the mitochondrial DNA of a human cell.
- Germ Cells- Reproducing cells of the body, sperm cells in males and eggs in females are the germ cells.
- Hematopoiesis – Formation of blood cellular components which is derived from hematopoietic stem cells (see blood cells)
- Homeostasis – Process of self-regulation to maintain internal steady state condition for the body's optimal functioning.
- Immunohistochemistry – A method of detecting antigens in a cell of a tissue section by exploiting functions of antigen-antibody binding. Antigens of the cell are detected by an anti-antigen antibody that is labeled with an enzyme of fluorophores. Visualization of chromogen (in the case of enzyme-labeled antibody) or fluorophores indicate the presence of specific antigens in the cell.
- Immortal DNA strands – The oldest nuclear DNA molecules in the chromosomes that are non-randomly retained by asymmetrically self-renewing tissue stem cells. They were first postulated to exist by Cairns in 1975.
- iPSCs – Induced pluripotent stem cells are bioengineered ESC-like cells produced by reprogramming somatic cells with pluripotent transcription factors.
- Kinetic Stem Cell (KSC) Counting – A method for determining the specific fraction or dosage of tissue stem cells based on monitoring how the produce progenitor and terminally-arrested cells in culture.
- Label Retention – This technique was previously used to identify slow-cycling cells or tissue stem cells with non-random chromosome segregation. A label like bromodeoxyuridine (BrdU) is once incorporated into the DNA of dividing cells, including stem cells. Retention of the label by cells after successive division in the absence of the label allows the identification of label-retaining cells (LRC). If LRCs are shown independently to be actively cycling, they are defined to be tissue stem cells.
- Macrophage – A type of blood cell that has phagocytic activity. When they reach a specific tissue location, monocytes can be induced to mature into macrophages.
- Mesenchymal Stem Cells (MSCs) – Multilineage stromal cells that have the potency to self-renew and differentiate into many cell types. MSCs can be isolated from various tissues, including bone marrow, adipose tissue, placenta, umbilical cord blood, endometrium, and others.
- Microvesicles – Microvesicles (MVs) are the vesicular structures shed off by the cells through blebbing of the plasma membrane. MVs are typically in the range of 0.1-1.0 µm in diameter.
- Morphology – Refers to a cell's size, shape, and structure.
- Neonatal – Newly born
- Neuron – Also called a nerve cell- is the fundamental brain and nervous system unit. The nervous system is made up of neurons.
- Newt – A newt is a salamander- amphibian
- Non-random Chromosome Segregation – A form of chromosome segregation specific to asymmetrically self-renewing tissue stem cells. Instead of new and old chromosomes having an equal likelihood of going to either new cell, the stem cell always gets the chromosomes with the older DNA.
- Organelle – Structures within the cells surrounded by a membrane and has a specific function are called cell-organelle.
- Paracrine – A type of cellular signaling where the signal (biomolecules) produced in a cell induces changes in nearby cells.
- Perinatal – Associated with birth; referring to the gestational tissues, the placenta, umbilical cord, and amnion sac.
- Phagocytosis – A process in which cells use their plasma membrane and cytoplasmic to engulf large particles like cell debris or bacteria. The formation of an internal structure with large particles inside the cell is called a phagosome.
- Postnatal – Occurring after birth
- Prenatal - Before birth
- Progenitor Cell – The progeny of stem cells with lesser potency than stem cells but higher potency than terminally differentiated cells are called progenitor cells. They are also called “transiently-amplifying cells” due to their high proliferation capacity.
- Regeneration – The process of restoring or replacing damaged/injured cells with functional ones is regeneration. Stem cells have regeneration power to replace or repair damaged cells with new or functional cells.
- Somatic Cell- All other cells of the body except germ cells (egg and sperm) are called somatic cells.
- Tunneling Nanotubes (TNT) – A membranous elongation of cells that connect each other to mediate trafficking materials (organelles, biomolecules, bioactive peptides, ions, and other cues) across them.
- Totipotent – Stem cells that can give rise to any cell type or a complete embryo, including embryonic and extra-embryonic tissues.
- Transcription – The process of formation of RNA from the DNA.
- Choudhary, R., 2014. Mammary stem cells: expansion and animal productivity. J Anim Sci Biotechnol 5, 36.
- Choudhary, R.K., and Capuco, A. V., 2021. Expression of NR5A2, NUP153, HNF4A, USP15 and FNDC3B is consistent with their use as novel biomarkers for bovine mammary stem/progenitor cells. J Mol Histol. 52(2), 289-300.
- Choudhary, R.K., Li, R.W., Evock-Clover, C.M., and Capuco, A. V., 2013. Comparison of the transcriptomes of long-term label retaining-cells and control cells microdissected from mammary epithelium: an initial study to characterize potential stem/progenitor cells. Front Oncol 3, 21.
- He, X., Hong, W., Yang, J., Lei, H., Lu, T., He, C., Bi, Z., Pan, X., Liu, Y., Dai, L., Wang, W., Huang, C., Deng, H., and Wei, X., 2021. Spontaneous apoptosis of cells in therapeutic stem cell preparation exerts immunomodulatory effects through release of phosphatidylserine. Signal Transduction and Targeted Therapy. 6(1): 270.
- Huang, Y., Bai, Z., and Zhang, K., 2022. A new insight for stem cell therapy: apoptotic stem cells as a key player. Signal Transduction and Targeted Therapy.7, 299.
- Lin, C.S., Xin, Z.C., Dai, J., and Lue, T.F., 2013. Commonly used mesenchymal stem cell markers and tracking labels: Limitations and challenges. Histol Histopathol 28, 1109–1116.
- Merok, J.R., Hatch, N.L., Tunstead, J.R., Sherley, J.L., Powers, M.J., Griffith, L.G., Panchalingam, K., Crane, G.G., and Lee, H.-S., 2003. Clonal expansion of adult rat hepatic stem cell lines by suppression of asymmetric cell kinetics (SACK). Biotechnol Bioeng 83, 760–771.
- Orozco-Fuentes, S., Neganova, I., Wadkin, L.E., Baggaley, A.W., Barrio, R.A., Lako, M., Shukurov, A., and Parker, N.G., 2019. Quantification of the morphological characteristics of hESC colonies. Scientific Reports 2019. 9, 17569.
- Panchalingam, K., Jacox, L., Cappiello, B.D., and Sherley, J.L., 2020. Non-random sister chromatid segregation in human tissue stem cells. Symmetry (Basel) 12(11). 1868.
- Pang, S.H.M., D’Rozario, J., Mendonca, S., Bhuvan, T., Payne, N.L., Zheng, D., Hisana, A., Wallis, G., Barugahare, A., Powell, D., Rautela, J., Huntington, N.D., Dewson, G., Huang, D.C.S., Gray, D.H.D., and Heng, T.S.P., 2021. Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nature Communications. 12, 6495.
- Sherley, J.L., Daley, M.P., and Dutton, R.A., 2022. Validation of Kinetic Stem Cell (KSC) counting algorithms for rapid quantification of human hematopoietic stem cells. J Stem Cell Ther Transplant 6, 29–37.
- Yang, F., Zhang, Yanbin, Liu, S., Xiao, J., He, Y., Shao, Z., Zhang, Yuhui, Cai, X., and Xiong, L., 2022. Tunneling nanotube-mediated mitochondrial transfer rescues nucleus pulposus cells from mitochondrial dysfunction and apoptosis. Oxid Med Cell Longev. 2022. 3613319.
- Zhang, L.N., Zhang, D. di, Yang, L., Gu, Y.X., Zuo, Q.P., Wang, H.Y., Xu, J., and Liu, D.X., 2021. Roles of cell fusion between mesenchymal stromal/stem cells and malignant cells in tumor growth and metastasis. FEBS J 288(5), 1447–1456.
- https://stemcells.nih.gov/
- https://stemcells.nih.gov/info/basics/stc-basics
- OpenAI. (2023). ChatGPT (July 10 version) [Large language model]. https://chat.openai.com/chat









